Congratulations to Genentech/Roche-ImmunoGen on the FDA Approval of Kadcyla (Ado-Trastuzumab Emtansine), an Antibody-Drug Conjugate for Her2-Positive Metastatic Breast Cancer
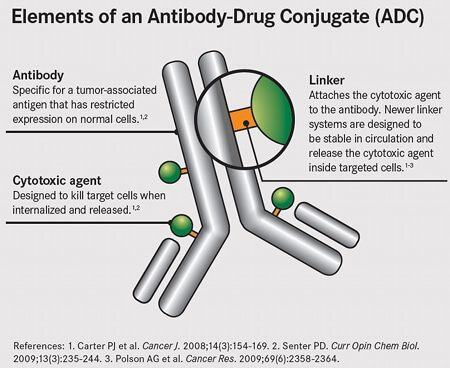
Antibody-Drug Conjugates (ADCs) combine the best of Nature's capacity to rapidly evolve specificity with Science's capacity to deliver specifically designed functionality.
There are fascinating, unique ADC SAR (Structure Activity Relationship) informatics representation and optimization challenges when one considers the whole problem. Biological sequence and small molecule structural components together create the therapeutic fingerprint. From an information complexity perspective, ADCs cover a very large, multidimensional space that can be combed across multiple axes. The challenge (which is a synonym for opportunity) is to co-optimize, and co-analyze, biological and chemical components. Often they are considered separately, ideally they should be evaluated synergistically.
Given the relative immaturity of ADC informatics, one can assume that our tools and understanding are in their infancy.
In response to this emerging complexity, Accelrys has developed SCSR (Self-Contained Sequence Representation) and the Pistoia Alliance promotes Pfizer's HELM (Hierarchical Editing Language for Macromolecules) in an active project. These technologies provide data dictionaries for dealing with unnatural (or unconventional) functionality in lieu of natural building blocks in (bio)-polymers. Disclosure: CDD is an Accelrys ISV partner and CDD financially supports the non-profit Pistoia Alliance at the member and director levels.
More on this new ADC approval from Genentech.
P.S. ADC research which was already hot, now will accelerate. The ADC approach, in light of Genentech's exciting FDA approval and the ADC approved by Seattle Genetics (Adcentris, a conjugate targeting Hodgkin's lymphoma, several types of T-cell lymphoma and other hematologic malignancies), has certainly come of age. Companies such as Redwood Biosciences, Sutro Biopharma, Ambrx, and Allozyne use site-specific conjugation technology to incorporate a nonnatural amino acid into the antibody and allow precise placement of the drug payload.
Other posts you might be interested in
View All Posts
CDD Vault Updates
4 min
April 25, 2025
CDD Vault Update (April 2025 #3): AI+ Folding and Docking
Read More
CDD Vault Updates
10 min
April 18, 2025
CDD Vault Update (April 2025 #2): Pharmacokinetic (PK) and Michaelis-Menten Kinetics (Km/Kd) Curve Fit Equations, Donut Charts, TIFF Image Previews, and Parallel Reactions
Read More
CDD Blog
3 min
April 14, 2025
Let’s Talk Security - Why a Bug Bounty May Be More Valuable Than a Penetration Test
Read More


