Like many around the world, we have been watching the devastating effects of the Ebola Virus in West Africa. We have been impressed by the important contributions of the health organizations and medical workers that have served patients at great personal risk to themselves and brought the disease under some degree of control. Especially during a crisis, seamless effective collaborations are critical. Collaboration can catalyze the communication of current knowledge and coordinate future action – whether for drug repurposing or de novo drug discovery. We wondered, how can those of us in drug discovery contribute in some way? We have found considerable interesting published results regarding molecules showing some activity against the Ebola Virus, including a number of FDA-approved drugs, and made them available as a public data set in CDD Vault for the scientific community. (See references Madrid et al. and Johansen et al.)
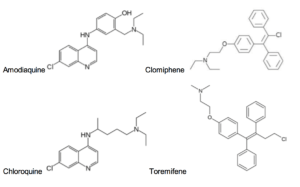
FDA-approved active against Ebola Virus in vitro
A recent pharmacophore based on these compounds was proposed to closely match the pharmacophore for ligands for the Ebola Viral protein 35 (VP35). Follow-up docking studies suggest that these compounds are likely to provide favorable inhibitory interactions with this receptor. VP35 is a cofactor in the RNA polymerase transcription complex, and helps the virus evade the immune response by blocking activation of the interferon regulatory factor 3, which is required for the induction of interferons alpha and beta. Thus, blocking VP35 should allow for an enhancement of the host immune response to the Ebola Virus.
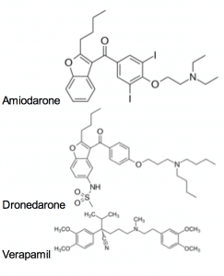
Another recent study has highlighted the ability of three clinically approved channel blockers to inhibit the Ebola virus cellular entry. These drugs: amiodarone, dronedarone, and verapamil, were given in vitro at concentrations that are reached in humans, and were effective against a number of filoviruses. The authors hypothesize that these drugs may act by disrupting late endosomal processing or by disrupting calcium signaling that is required for viral entry.
Molecules shown to inhibit Ebola Virus entry
None of these compounds were designed to target Ebola. Amodiaquine and chloroquine are antimalarials. Clomiphene and toreminfene are estrogen receptor modulators. Amiodarone, dronedarone, and verapamil are anti-arrhythmics. However, they are all orally bioavailable and safe for humans. Thus, without an approved treatment, these drugs may represent a fast and feasible option for preventing the spread and mortality associated with Ebola Virus in a large population.
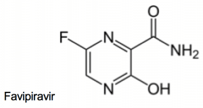
Another drug, favipiravir, which is undergoing phase 3 clinical trials in the US for influenza and is approved in Japan, has shown promising efficacy against Ebola Virus in mice. Faviparavir is thought to act by inhibiting the viral RNA-dependent RNA polymerase selectively and has demonstrated activity against a number of viruses. At least one Ebola patient, who has since recovered, was given favipiravir, and Japan has offered to supply it to the World Health Organization.
Favipiravire effective against Ebola Virus in Mice
Beyond these FDA-approved drugs and drugs in clinical trials there are many other compounds that have shown activity in vitro or in vivo. While not ready for in human use, they may present an attractive starting point to be refined in a drug discovery effort. An experienced medicinal chemist recently evaluated these compounds to identify potentially problematic moieties, and has now made this data set of 55 compounds with reported Ebola data publicly available here. Of course, collaborative tools like CDD Vault can be used to store the small molecule data for the Ebola Virus in the same way as done to catalyze progress in other neglected and commercial drug discovery collaborations.
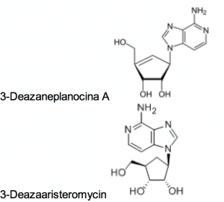
Beyond these FDA-approved drugs, 3-Deazaneplanocina A and 3-Deazaaristeromycin, potent inhibitors of the cellular enzyme s-adenosylhomocystein hydrolase that is required for interferon production in infected cells, have shown potent activity against Ebola Virus. These compounds also have potent anti-cancer activities, but have not yet progressed into clinical trials. While not ready for in human use, they may present an attractive starting point to be refined in a drug discovery effort.
Oligonucleotide analogs with activity against Ebola Virus
It is a great honor to work on improving the quantity and quality of human health. We also need to have the humility to realize when social, political, and economic adjustments can help as much as scientific knowledge. The Gates Foundation and others have emphasized the importance of healthcare infrastructure. There are a great many lessons from this outbreak, some related to drug discovery and others related to the infrastructure needed to improve the rate of response to the next risk.
Other posts you might be interested in
View All Posts
Events
11 min
March 27, 2025
Collaborative Drug Discovery's Inaugural Canadian User Group Meeting
Read More
CDD Blog
5 min
March 21, 2025
Drug Discovery Informatics for Big Pharma: Key Webinar Insights
Read More
CDD Vault Updates
3 min
March 19, 2025
CDD Vault Update (March #2 2025): Macromolecule Atomistic Rendering, AI Datasets, Import Inventory Locations, Larger Inventory Boxes, Dark Mode
Read More