From both big pharma and more specialized drug repositioning companies presenting at the Drug Repositioning Conference in San Francisco last week, it became clear that if one can get an IP hook, the strategy allows one to quickly create significant monetizable value with a relatively small investment.
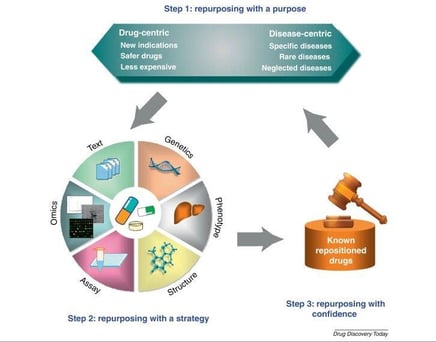
from: http://dx.doi.org/10.1016/j.drudis.2012.08.005
To highlight two memorable examples:
- Cephalon bought bdc for $70m with just an “animal use patent” for sciatica to compete w/ Amgen’s Embrel
- Adolor repositioned the potential anti-depressant Org4428 that stalled at phase 3 for depression as a non-opioid for pain, received a fast-track use patent, and successfully partnered the program with Organon (which was acquired by Schering-Plough, now Merck).
Bottom line is if you can show differentiation, capture IP, and show a cost-benefit commercial path to market, then repurposing is a fast (perhaps the fastest) route to an approved drug.
Barry Bunin chaired a webinar panel with Chris Lipinski, David Cavalla and Noel Southall. Download the talks below. We’ll provide full talk transcripts and archive these materials on the CDD Resources page for easy reference.
- David Cavalla slides and transcript
- Barry Bunin talk, slides and transcript
- Chris Lipinski talk, slides and transcript
- Noel Southall talk, slides and transcript
Tag(s):
Other posts you might be interested in
View All Posts
CDD Vault Updates
4 min
April 25, 2025
CDD Vault Update (April 2025 #3): AI+ Folding and Docking
Read More
CDD Vault Updates
10 min
April 18, 2025
CDD Vault Update (April 2025 #2): Pharmacokinetic (PK) and Michaelis-Menten Kinetics (Km/Kd) Curve Fit Equations, Donut Charts, TIFF Image Previews, and Parallel Reactions
Read More
CDD Blog
3 min
April 14, 2025
Let’s Talk Security - Why a Bug Bounty May Be More Valuable Than a Penetration Test
Read More


